Grant-in-Aid for Scientific Research (S)
- 【Research Project Number】16H06384
- 【Researcher Number】20227060
- 【Term of Project】2016-2020
- 【Budget Allocation】123,300,000 JPY
- 【Research Area】Pharmaceutical Chemistry, Synthetic Organic Chemistry
- 【Keyword】Synthetic Chemistry, Catalyst, Glycoside, Peptide, Large- and Medium-size Molecule
- 【Contact Information】takemoto@pharm.kyoto-u.ac.jp
- 【Title of Project】Innovative catalysts for the synthesis of large- and medium-sized molecules bearing glycopeptides
- 【Principal Researcher】Yoshiji Takemoto(Kyoto University, Graduate School of Pharmaceutical Sciences, Professor)
Purpose and Background of the Research
There has been a recent increase in the number of biomedicines being developed based on antibodies and nucleic acids. Most of the approved biological therapeutic agents are manufactured using biological methods because there are very few chemical tools available. However, there are several issues associated with the biological processes currently used, including (1) they cannot be used to prepare structurally pure compounds; (2) biological synthesis processes are expensive; and (3) the development of site-selective chemical modifications is challenging.
To develop efficient drug production processes, as well as therapeutic agents capable of contributing to life science research, there is an urgent need to establish facile, economical, and scalable synthetic methods for the preparation of chemically modified glycopeptides. However, most routine chemical reactions require the addition of excessive amounts of expensive and toxic dehydrating reagents, which leads to large amounts of chemical waste.
The aim of this project is to establish innovative synthetic methods using new catalysts for large- and medium-sized molecules via the site-selective modification of existing compounds composed of amino acids and monosaccharides.
Research Methods
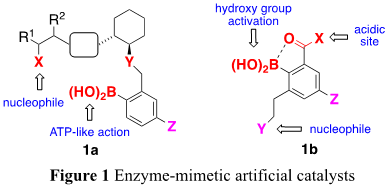
The sustainable synthesis of glycopeptides requires the catalytic formation of peptide and glycosyl bonds without using dehydrating agents. Towards this goal, we have designed a series of new catalysts based on the mechanisms associated with the non-ribosomal peptide synthetase- and glycosidase-catalyzed preparation of peptide and oligosaccharides, respectively (Figure 1).
Catalyst 1a consists of an arylboronic acid, which could activate the carboxylic acid moiety of an amino acid substrate, and a thiol group, which could act as a nucleophile for the subsequent formation of a thioester. We intend to investigate the catalytic efficiency of 1a in (1) the aza-Michael addition for the synthesis of N-alkoxy-α-amino acids and (2) the formation of peptides without any dehydrating agents. It is envisaged that catalyst 1b could be used to selectively activate the diol units found in monosaccharides using an arylboronic acid. By tuning the second functional group, we will be able to optimize this catalyst for (3) activation-free glycosylation processes and (4) the divergent synthesis of oligosaccharides.
Expected Research Achievements and Scientific Significance
This research could result in the development of an environmentally benign manufacturing technology for the preparation of glycopeptides.
The development of new methods for the site-selective modification of glycopeptides will have a considerable impact on the life sciences.