1.Multi-Functional Organocatalysts for Synthesizing Biologically Active Compounds
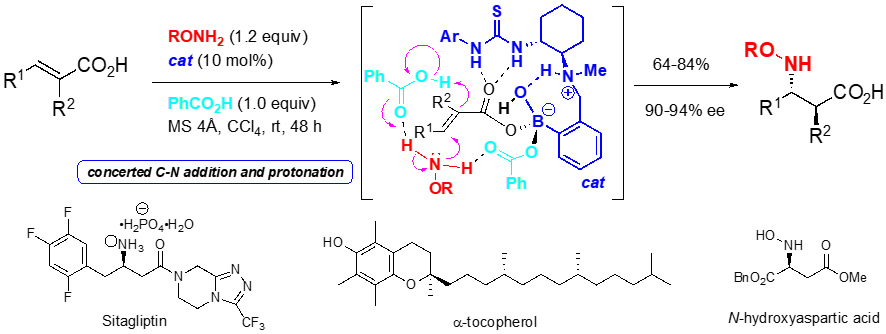
Thiourea-aminoboronic acid hybrid catalysts
Carboxylic acids are generally utilized as Brønsted acids and oxygen nucleophiles in organic synthesis. However, a few asymmetric reactions have used carboxylic acids as electrophiles. We have developed chiral thioureas (cat) bearing both arylboronic acid and tertiary amine to promote the hetero-Michael addition of BnONH2 and phenol to α,β-unsaturated carboxylic acids. Synthetic versatility of the reactions is demonstrated by the accomplishment of the asymmetric synthesis of key intermediates in pharmaceutically important molecules (J. Am. Chem. Soc. 2018, 140, 12216).
2.Total Synthesis of Biologically Active Natural Products
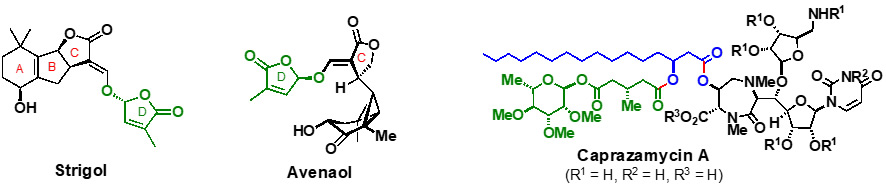
(1) Avenaol
Avenaol, isolated from the allelopathic plant black oat, is the first C20 germination stimulant related to strigolactones. It consists of a bicyclo[4.1.0]heptanone skeleton containing a cyclopropane ring bearing three main chains projecting in the same direction (i.e. all-cis-substituted cyclopropane). The key factors in the success of the first total synthesis include the Rh-catalysed intramolecular cyclopropanation of an allene, an Ir-catalysed stereoselective double-bond isomerisation, and the differentiation of two hydroxymethyl groups (Nat. Commun. 2017, 8, 674).
(2) Caprazamycin A
Caprazamycin A has significant antibacterial activity against Mycobacterium tuberculosis (TB). We have achieved the first total synthesis of caprazamycin A, and the salient features of the synthesis are a) the scalable preparation of the syn-β-hydroxy amino acid with a thiourea-catalyzed diastereoselective aldol reaction, and b) construction of a diazepanone with an unstable fatty-acid side chain. This report provides a route for the synthesis of related liponucleoside antibiotics with fatty-acid side chains (Angew. Chem. Int. Ed. 2015, 54, 3136).
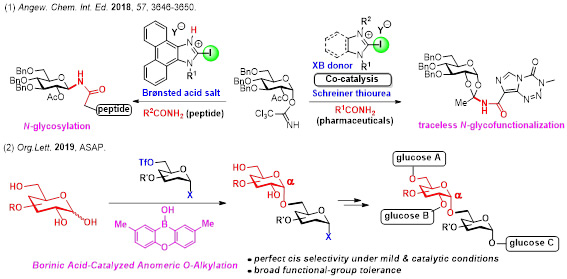
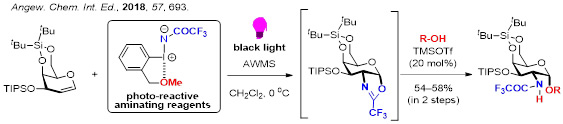
3. Direct, Efficient, and Selective Glycofunctionalization and Oligosaccharide Synthesis
Sugar moieties are known to impart hydrophilicity to molecules, and alter the higher order structure and bioavailability of molecules. To date, a variety of synthetic methodologies for chemical glycosylation have been developed, but there still remains to be improved concerning the issues including 1) direct N-glycosylation of amides, 2) oligosaccharide synthesis without protecting groups, and 3) glycosylation with perfect control of the stereochemistry of the anomeric position. In order to solve these problems, we have developed several unique glycofunctionalization using newly developed catalysts and reagents.
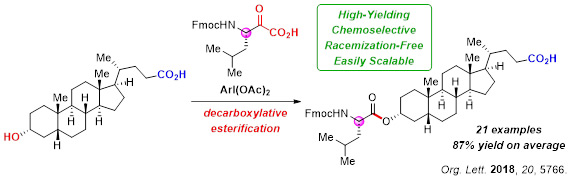
4.Chemoselective, Racemization-Free Esterification Using Hypervalent Iodine(III)
Selective chemical modification by the introduction of amino acid or peptide structures would be a powerful tool in medicinal chemistry, and moreover, from the aspect of providing a practical coupling method, the preservation of stereochemical information would be critically important.
An α-ketoacid can be converted into a reactive acylating agent by treatment with hypervalent iodine(III) species, and in doing so, we discovered a novel decarboxylative acylation of alcohols that affords a variety of esters in excellent yields. The esterification has been applied to a sterol bearing a free carboxylic acid and shows unique chemoselectivity.
5. New Reagents for Chemoselective Transformation without Using Activator
Chemical reactions without using catalysts or promotors have significant potential to the late-stage functionalization of bioactive compounds and prodrug synthesis. We have designed and developed N-acyliminoiodinanes, which can be activated by photoirradiation at 370 nm, enabling reaction with various silyl enol ethers to give α-aminoketone derivatives in good to high yields. N-Acyliminoiodinanes are characterized for the first time by X-ray structural analysis. The ortho-methoxymethyl group and the carbonyl oxygen coordinate to the iodine atom of the iminoiodinane.